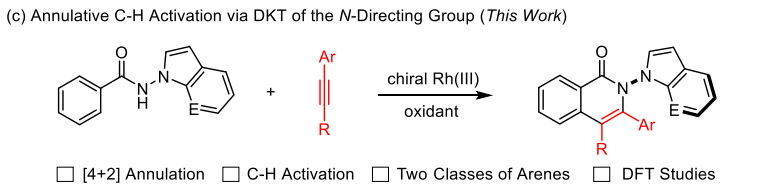
N-N axially chiral biaryls represent a rarely explored class of atropisomeric compounds. We hereby report rhodium-catalyzed enantioselective [4+2] oxidative annulation of internal alkynes with benzamides bearing two classes of N-N directing groups. The coupling occurs under mild conditions via NH and CH annulation through the dynamic kinetic transformation of the directing group and is highly enantioselective with good functional tolerance. Computational studies of a coupling system at the DFT level has been conducted, and the alkyne insertion was identified as the enantio-determining as well as the turnover-limiting step.
(Chem. Sci., 2023, DOI: 10.1039/D3SC02800C)
Congratulations to Xiaohan Zhu and Yishou Wang. Special thanks also go to our collaborators.