Expedient Synthesis of Axiallyand Centrally Chiral Diaryl Ethers via Cobalt-Catalyzed Photoreductive Desymmetrization
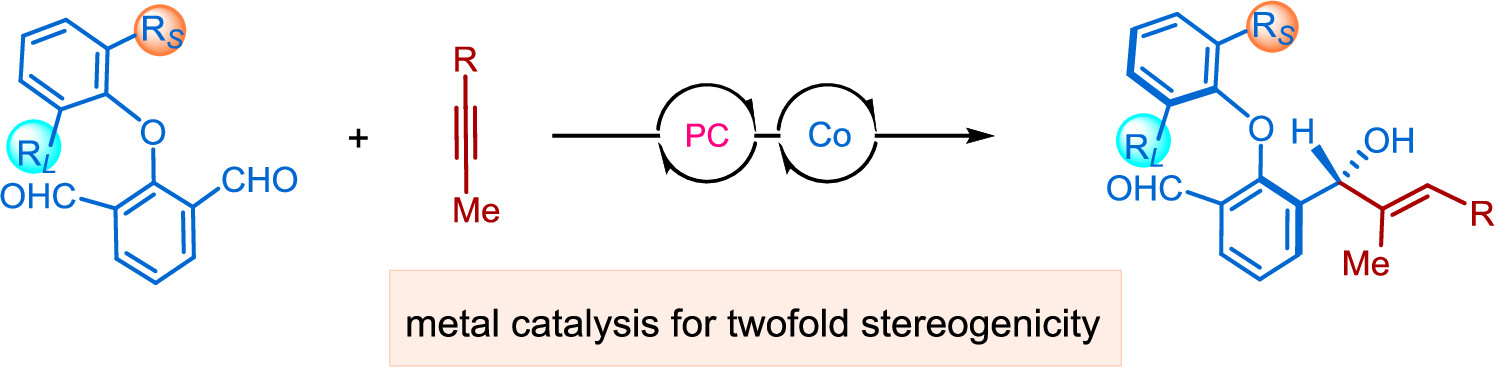
Axiallychiral diaryl ethersmake up a uniqueclass of atropisomers bearing restricted rotation about the C−O bond.Methodsfor the expedient synthesis of axiallychiral diaryl ether-based structures have been largely underdeveloped.Herein,we developed an efficientmetal-catalyzed desymmetrizationstrategyto unveil the formation of axially and centrallydual chiral diarylethers in high diastereo-and enantioselectivity.The protocolleveragescobalt-catalyzed photoreductive enantioselective couplings of dialdehyde and alkyneto deliverdual stereogenicity,and the diaryl ether scaffoldis equipped with useful synthetic handles including formyl,hydroxyl,and allyl groups,as has been demonstratedin the synthesisof a chiral carboxylicacid as a potentialchiral ligand in asymmetric catalysis. (Cooperated with the team of Shandong University)
(ACS Catal. 2024, 14, 4638−4647)
Congratulations to Yishou Wang. Special thanks also go to our collaborators.