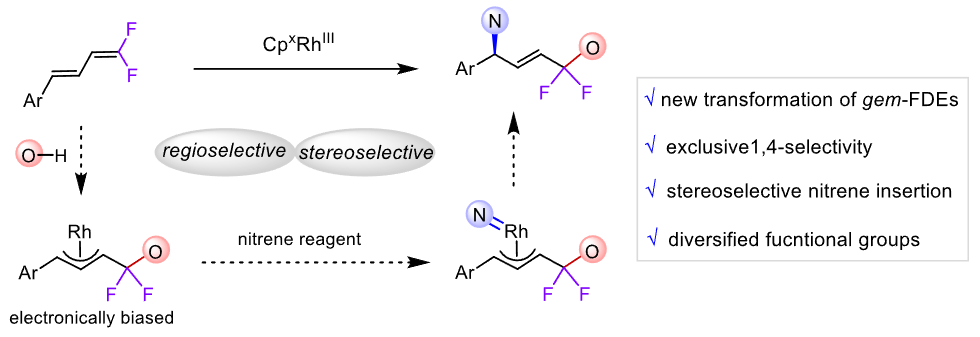
The incorporation of fluorine atoms in organics conduces to improvement of their bioactivity and lipophilicity. Catalytic functionalization of gem-difluorodienes represents one of the most straightforward approaches to access fluorinated alkenes. However, the regio- and enantioselective oxyamination of gem-difluorodienes remains untouched. Herein, we report asymmetric 1,4-oxyamination of gem-difluorodiene via chiral rhodium-catalyzed three-component coupling with readily available carboxylic acid and dioxazolone to forge a gem-difluorinated and enantioenriched 1,4-amino alcohol derivatives. Our asymmetric protocol exhibits high 1,4-regio- and enantioselectivity with utility in the late-stage modification of pharmaceuticals and natural products. Stoichiometric experiments provide evidences for the π-allylrhodium pathway. Related oxyamination was also realized when trifluoroethanol was used as an oxygen nucleophile. (Cooperated with the team of Shandong University)
Angew. Chem. Int. Ed. 2023, e202305669.
Congratulations to Heng Song. Special thanks also go to our collaborators.