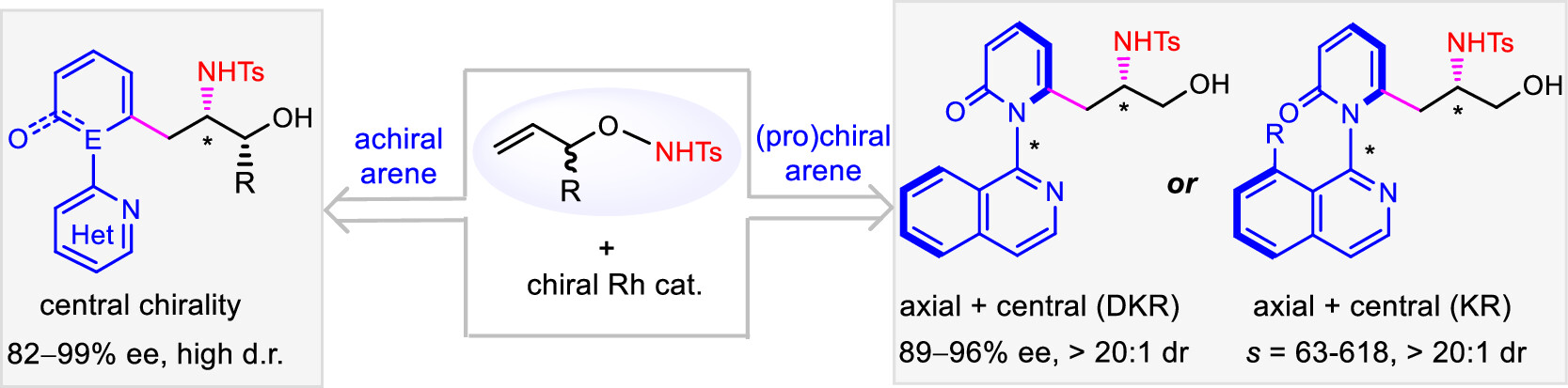
Difunctionalization of olefins offers an attractive approach to access complex chiral structures. Reported herein is the design of N-protected O-allylhydroxyamines as bifunctional olefins that undergo catalytic asymmetric 1,2-carboamidation with three classes of (hetero)arenes to afford chiral amino alcohols via C–H activation. The C═C bond in O-allylhydroxyamine is activated by the intramolecular electrophilic amidating moiety as well as a migrating directing group. The asymmetric carboamidation reaction pattern depends on the nature of the (hetero)arene reagent. Simple achiral (hetero)arenes reacted to give centrally chiral β-amino alcohols in excellent enantioselectivity. The employment of axially prochiral or axially racemic heteroarenes afforded amino alcohols with both axial and central chirality in excellent enantio- and diastereoselectivity. In the case of axially racemic heteroarenes, the coupling follows a kinetic resolution pattern with an s-factor of up to >600. A nitrene-based reaction mechanism has been suggested based on experimental studies, and a unique mode of induction of enantio- and diastereoselectivity has been proposed. Applications of the amino alcohol products have been demonstrated. (Cooperated with the team of Shandong University)
(https://doi.org/10.1021/jacs.3c01162)
Congratulations to Ruijie Mi. Special thanks also go to our collaborators.